How a Tiny Chip Restored Reading to the Blind — The PRIMA Breakthrough That Could Redefine Vision
A new PRIMA retinal implant — a 2×2mm light-powered microchip paired with AR glasses — restored readable central vision for the majority of patients in a recent multi-center trial. Here’s how it works, what the data show, who can benefit, and why this matters for AI, prosthetics and health systems.
Intro — a tiny chip, a huge change
On October 20, 2025, the medical world got a jolt: researchers reported that a palm-sized retinal microchip called PRIMA restored central reading vision to many patients who had lost it from advanced dry age-related macular degeneration (geographic atrophy). In a multi-center clinical trial published alongside press releases and coverage, most participants could again read letters and short words using the prosthetic system — a result that, until now, many clinicians regarded as aspirational.
The PRIMA retinal implant is a 2×2 mm photovoltaic microchip paired with AR glasses that restores readable central vision for many patients with advanced dry AMD.
This isn’t science fiction. It’s a combination of microsurgery, optics, wearable processing, and precise stimulation of retinal tissue — a human-scale victory for neuroprosthetics. In this article we unpack the technology, the trial data, who benefits, the risks and rollout hurdles, and why this matters beyond ophthalmology.
PRIMA retinal implant — how the device works and who benefits
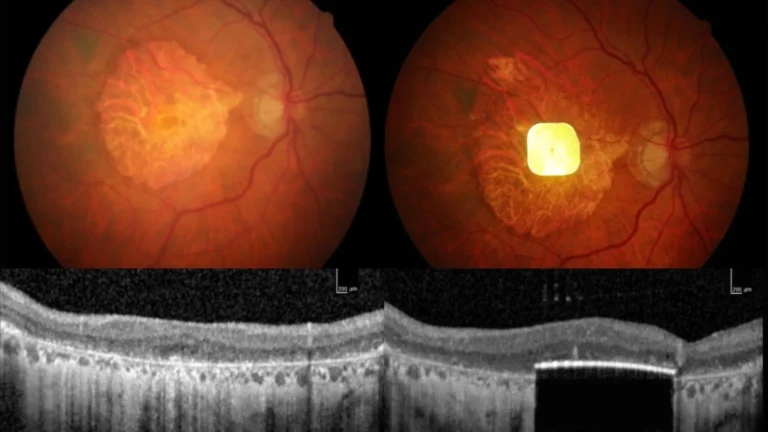
What is PRIMA and how does it work?
Short version: PRIMA is a 2mm × 2mm implant, placed beneath the central retina, that converts patterned light into electrical stimulation the remaining retinal tissue can interpret. The patient wears augmented-reality (AR) glasses that capture and preprocess video; the processed signal is transmitted to the tiny implant, creating a prosthetic central image while the eye’s peripheral vision remains natural. In the trial, the PRIMA retinal implant enabled patients to recognise letters and short words by converting patterned light into safe electrical stimulation of remaining retinal cells.
Key components:
Microchip (the implant): Ultra-thin photovoltaic array implanted under the retina.
Wearable processor/AR glasses: Capture, preprocess and optimize images (contrast, brightness, edge detection).
Surgical delivery: Vitrectomy and subretinal insertion through a micro incision (procedure ~under two hours in experienced hands).
Because the chip is powered by light (photovoltaic), there’s no bulky battery inside the eye — the glasses supply the light pattern and the implant converts it to signals that stimulate retinal neurons.
The trial: who, what, and how well did it work?
Study design (high level):
Multi-center international trial co-led by major vision centers. Patients enrolled had advanced atrophic dry AMD with central vision loss (only peripheral vision remained). The trial tested whether prosthetic stimulation could restore meaningful central vision (e.g., letter recognition).
Top-line results (reported):
Participants: Tens of patients across many sites (trial reported 38 patients in press coverage).
Reading restored: A large majority regained the ability to recognise letters and read short words; trial publications report that ≈84% of participants could read letters, with an average reading level of about five chart lines (a clinically meaningful gain).
What “reading” means here: Not fluent novels — yet — but the ability to identify letters, numbers, and short words that patients had lost for years. For many, that’s a radical improvement in daily independence, mood and quality of life.
Who benefits — selection & eligibility
Not every patient with AMD is a candidate. The PRIMA implant targets those with geographic atrophy (advanced dry AMD) where the central macula has degenerated but peripheral retina remains. Important selection criteria:
Stable peripheral vision (to retain mobility/navigation).
Sufficient retinal structure outside the atrophic lesion to interface with stimulation.
General surgical fitness (vitrectomy is required).
Realistic expectations: prosthetic central vision differs from natural sight — it’s lower resolution and requires training/rehab.
Clinicians emphasise patient counselling: the aim is functional improvement (reading, facial recognition, better independence), not full restoration to pre-disease acuity.
The surgical pathway and rehab
Surgery: Standard vitreoretinal techniques — the microchip is inserted under the central retina via a small incision after vitrectomy. Experienced vitreoretinal surgeons (trial sites included Moorfields and key university centers) report the operation can be performed safely in under two hours.
Rehabilitation: Postoperative training is crucial — patients learn to interpret prosthetic input (contrast, edges) and combine artificial central signals with natural peripheral vision. Visual rehabilitation programs typically run weeks–months.
Safety and risks
Surgical risks: Standard posterior segment surgery risks — infection, retinal detachment, hemorrhage.
Device risks: Implant migration, failure, or long-term tissue reaction (ongoing follow-up required).
Realistic risk/benefit: For patients with no central vision, the potential quality-of-life gains may outweigh risks, but broad adoption requires long-term safety and durability data.
The trial’s safety profile in published press notes was encouraging, but long-term surveillance (years) will determine durability and late complications.
How PRIMA compares with other approaches
There are several routes to artificial vision:
Retinal prostheses (PRIMA, others): Stimulate the retina directly (best when some retinal neurons remain).
Optical nerve or cortical implants: Direct stimulation of the optic nerve or visual cortex (useful when retina severely damaged).
Gene therapy / cell therapy: Replace or rescue photoreceptors before end-stage degeneration.
External vision aids (AR, AI assisted): Camera + software that augments low vision.
PRIMA’s advantage: minimal implant size, photovoltaic powering, and a demonstrated functional gain for end-stage dry AMD patients — a population with limited alternatives.
Cost, access and scale-up hurdles
Cost: Advanced devices plus surgery and rehab will be expensive initially. Reimbursement pathways (national health services, insurers) will dictate access.
Training: Surgeons and rehab teams need training; surgical volume will scale gradually.
Manufacturing & supply chain: Microfabrication, quality control, and AR hardware partnerships must scale.
Regulatory: NEJM publication and multi-center trials are a step; full regulatory adoption (FDA/EMA/UK MHRA) will require longer follow-up.
Clinicians and payers should view the PRIMA retinal implant as a hybrid medical-device plus software system — surgical skill, rehab and device-provenance matter as much as the chip itself.
Why this matters beyond eyes: prosthetics + AI integration
PRIMA is a nexus of hardware, optics, signal processing, and adaptive software. The AR glasses use algorithms (edge detection, contrast boosting) — essentially early AI assistance for vision. This trial signals that combining miniaturized hardware with smart processing can deliver functional neuroprosthetics. Expect similar patterns in:
Cochlear implants → auditory restoration scaling.
Brain-computer interfaces for speech (already promising in ALS).
AI-assisted prostheses for motor function.
The PRIMA story is a blueprint for future hybrid systems that couple implant hardware with wearable intelligence.
Ethical & practical questions
Equity: Who will be first to access these devices?
Informed consent: Patients must understand limitations and rehab needs.
Long-term monitoring: Implants raise questions about device updates, recalls, or compatibility with future AR systems.
What the near future looks like
Wider trials, longer follow-up, cost data, and regulator reviews in 2026–2028.
Iterations of PRIMA with higher pixel density, improved preprocessing algorithms, and more ergonomic wearables.
Integration with on-device AI to personalize stimulation patterns for each patient.
This is likely the start, not the end, of a wave of clinically useful neuroprosthetic devices.
Practical takeaways for patients and clinicians
If you have advanced dry AMD, ask retinal specialists whether trials or referral criteria apply.
For clinicians: begin preparing multidisciplinary rehab pathways (OT, low-vision specialists, tech support).
For payers and policy makers: early planning for coverage, training, and supply chains avoids bottlenecks once approvals arrive.
Audit for PRIMA retinal implant eligibility in advanced dry AMD pathways.
Sources & further reading
UPMC / University of Pittsburgh press release summarizing NEJM trial. EurekAlert!
ScienceDaily coverage: “Tiny AI-powered eye implant helps the blind see again.” SciTechDaily
ClinicalTrials/industry coverage summarizing device mechanics and trial scope. Clinical Trials Arena
New England Journal of Medicine — trial publication DOI referenced in press materials (see UPMC/EurekAlert press release). EurekAlert!
